The statement of the Food and Drug Administration or FDA regarding COVID-19 vaccination created a complication with the Biden administration’s mandate for having booster shots for everyone over 16. The FDA states that vaccination shot currently to the US people is good enough to provide enough protection against severe disease and COVID-19 without any additional dose.
An outside panel of scientific advisers will review the FDA report on Friday, along with a companion analysis from Pfizer and other information, as part of a discussion over who needs booster shots and when.
Chances are that booster shots will be recommended only for people in certain groups who have maximum chances of getting worsen such as medical staff or people 65 and older. However, data analyzed by FDA scientists the Pfizer-BioNTech single shot is not effective always in all cases according to infection and death rate. Even the third booster shot showed an adequate increase of neutralizing antibodies to protect the body against COVID-19.
Gregory Poland, director of the Mayo Clinic’s vaccine research group, said “To me, this is insufficient evidence to support a national booster program.” He also opined more data would be required to study to make a decision about the requirement of booster shots.
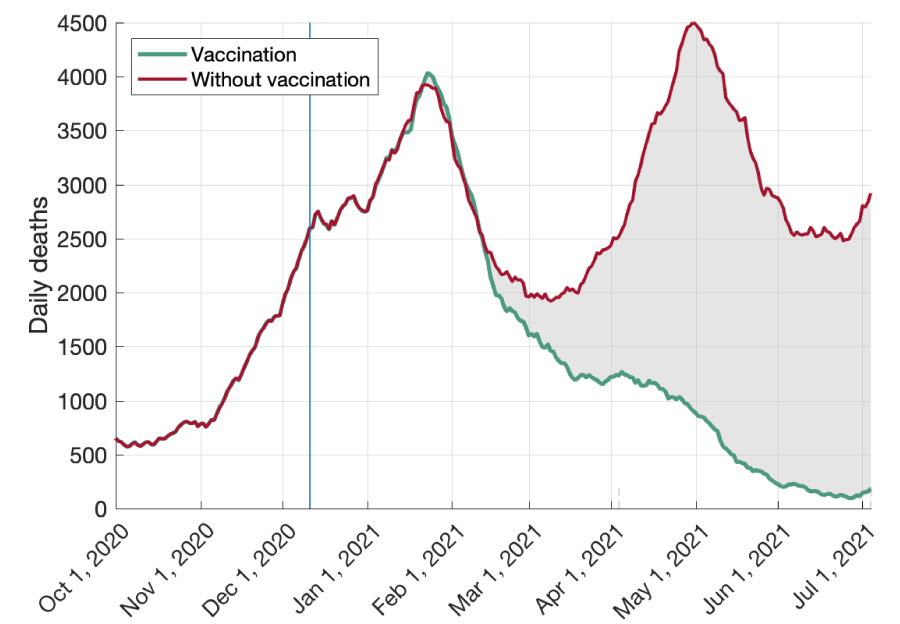
The Biden administration and drug companies confirmed the adequacy of the boosters if supply requires more units for wider distribution.
Bottom Line: Biden administration’s widespread drive for booster dosage became complicated as FDA says additional doses are not required for the general public to fight COVID-19.