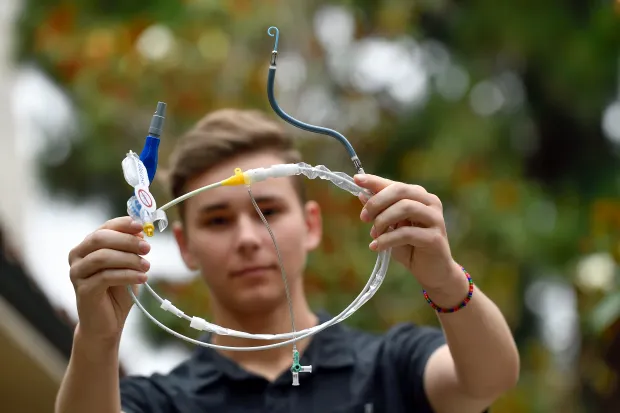
The US Food and Drug Administration (FDA) has issued the highest level alert about a heart pump associated with 49 deaths and 129 injuries.
Impella left-sided pumps are typically used to provide temporary support to a patient’s heart during risky procedures or following a severe heart attack.
However, the FDA cautioned that incorrect usage could lead to heart wall puncture, posing grave risks.

Manufacturers Respond
Manufacturer Abiomed has responded by releasing updated instructions for the pump’s use.
The FDA’s recent action, categorized as the most severe recall type, underscores the potential for severe injuries or fatalities if the device is misused.
While the affected pumps remain on the market, their utilization may result in significant adverse health effects, including hypertension and restricted blood flow, even death, the FDA said.
The recall pertains to 66,390 devices distributed in the US since October 2021.
Abiomed, now under Johnson & Johnson, clarified that the “notification is not a device removal, and Impella heart pumps remain on the market and available for patients.”